Molecular mechanisms of functional selectivity of atypical agonists at muscarinic receptors
Ectopic agonists represent a new class of drugs that bind out of the orthosteric site and display unique functional selectivity through mechanisms yet to be defined. We perform a detailed analysis of receptor activation induced by ectopic and classical agonists with the aim to reveal molecular mechanisms underlying functional selectivity.
Each of the five known subtypes of muscarinic acetylcholine receptors is involved in specific physiological functions. Drugs targeted at these receptors should therefore exhibit selective binding or functional selectivity among subtypes to achieve therapeutic effects without major side effects. Due to high homology of the orthosteric binding site among subtypes classical agonists display poor selectivity. Ectopic agonists like xanomeline or AC-42, however, represent a new class of drugs that bind somewhere else on the receptor and display unique functional selectivity through mechanisms yet to be defined. We propose a detailed analysis of receptor activation, oligomerization and conformational changes induced by ectopic and classical agonists with the aim to reveal molecular mechanisms underlying functional selectivity of the former class agonists. We will apply functional assays to determine receptor activation kinetics, and fluorescence resonance energy transfer in modified receptors to assess receptor conformational changes and oligomerization, and molecular modeling to verify obtained data. Knowledge of the molecular mechanisms of functional selectivity of atypical muscarinic agonists is indispensable for devising molecules acting as the selective agonists for pharmacotherapy of specific disorders.
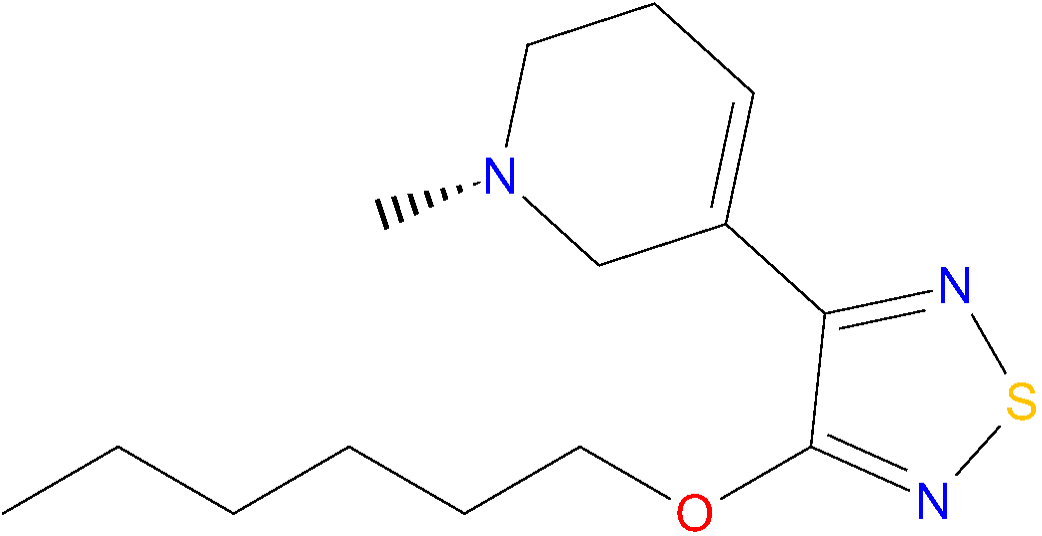
Chemical structure of xanomeline.
