The ability to transport K+ cations and hence the physiological role of Na+/H+ antiporters is influenced by the composition of their hydrophilic C-termini
It is important for every cell to constantly control the ionic composition of its internal environment (cytoplasm). To cope with the high salt concentrations (e.g. NaCl), cells must be able to eliminate sodium (Na+) cations. On the other hand, the export of potassium cations (K+) from cells is related to the regulation of intracellular pH and membrane potential. Na+/H+ antiporters are among transport systems that ensure the transport of Na+ and K+ out of the cells in exchange for protons in the cells of all eukaryotic organisms, from unicellular yeast to humans. For possible pharmacological intervention in the ion balance, it is necessary to discover, at the molecular level, how the structure of the antiporter impacts its functions.
Na+/H+ antiporters, like most membrane proteins, consist of a part located in the membrane and a part oriented to the cytoplasm - the hydrophilic C-terminus (see figure). Until now, it was assumed that only the transmembrane part determines which ions will be transported by the antiporter. In our new work, we demonstrate that the composition of the hydrophilic C-terminus of the antiporter is also important for determining substrate specificity (especially the ability to transport K+).
For the study, we used a single-cell model organism of eukaryotic cells - the yeast Saccharomyces cerevisiae, in which we expressed the Sod2-22 antiporter from the osmotolerant yeast Zygosaccharomyces rouxii, which efficiently exports only Na+ and Li+ cations, but not K+, from the cells. We found that replacing only one amino acid (introducing a negatively charged residue or removing a positively charged residue) in one of the conserved C-terminal regions (C3) enabled the antiporter to transport K+. Truncation or replacement of the C-terminal part of ZrSod2-22 with the C-terminus from another K+-transporting antiporter (S. cerevisiae Nha1 or Z. rouxii Nha1) also resulted in an antiporter with the capacity to export K+. This work provides a number of new insights into the relationship between the structure and function in the Na+/H+ antiporter family in eukaryotic cells.
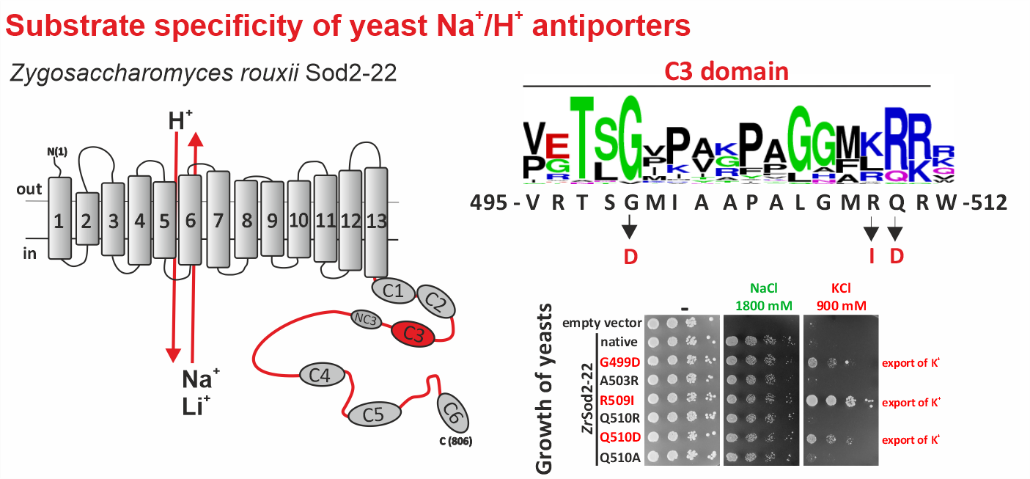
Left: Topological model of Z. rouxii Na+/H+ antiporter Sod2-22 with indicated conserved domains located in the hydrophilic C-terminus. Right: Mutations of particular residues in the conserved domain C3 changed the substrate specificity of the antiporter and enabled it to mediate the export of K+ (demonstrated by the growth of cells expressing these mutated versions in the presence of KCl).
Zimmermannova O., Velazquez D., Papouskova K., Prusa V., Radova V., Falson P., Sychrova H.: The hydrophilic C-terminus of yeast plasma-membrane Na+/H+ antiporters impacts their ability to transport K+. J Mol Biol. 436, 168443 (2024). IF = 5.6 DOI
