SPIM – Selective Plane Illumination Microscope
Our SPIM was constructed at the Dpt. Biomathematics with the support of Dpt. Cardiovascular Morphogenesis (Prof. Sedmera) by using instructions in OpenSPIM (openspim.org), which is an open access platform for applying and enhancing SPIM [1]:
[1] Pitrone P. G., Schindelin J., Stuyvenberg L., Preibisch S., Weber M.; Eliceiri K. W., Huisken J., Tomancak P. (2013) OpenSPIM: an open access light sheet microscopy platform, Nat. Methods 10, 598-599, 10.1038/nmeth.2507.
Manager: RNDr. Barbora Radochová, Ph.D., barbora.radochova@fgu.cas.cz
Deputy Manager: Ing. Martin Čapek, Ph.D., martin.capek@fgu.cas.cz
Technical parameters
The most suitable specimens for acquisition are the fixed ones that were made transparent by optical clearing. However, only water-based clearing protocols can be applied. We tested Scale, CLARITY and CUBIC up to now. The diameter of specimens can be circa in the range of 0.1-1.5mm.The specimens are embedded in a low melting point agarose inside a 1ml plastic syringe. Please, see preparation instructions:
Sample preparation (video; Carl Zeiss)
Sample preparation (PDF; Carl Zeiss)
Specimens are immersed into a sample chamber which is filled by water or clearing solution. Two acquisition setups are available:
- 4x dry Olympus objective; liquids applicable in the sample chamber – Scale, CUBIC, water.
- 10x, 20x water-dipping Olympus objectives; only water is applicable here!
The specimens are illuminated and optically sectioned by Coherent CUBE 490nm 52mW laser. The camera used is high sensitivity EMCCD Andor iXon3.
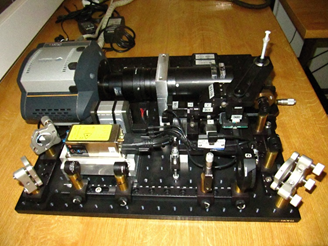
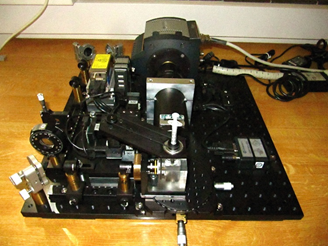
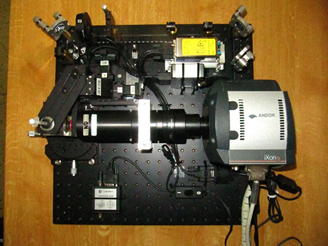
SPIM principle
The SPIM technology offers fast, optically-sectioning, minimally-invasive 3D acquisition of fluorescing specimen over time. It achieves that by focusing a thin laser light-sheet into the specimen, taking two-dimensional images of the illuminated slice with a perpendicularly positioned detector (CCD camera). Three-dimensional stacks are obtained by moving the specimen orthogonal to the light-sheet between consecutive images. By mounting the sample in a rigid medium, e.g. agarose, and hanging it into the sample chamber in front of the detection lens, it is possible to rotate the sample and collect 3D stacks from multiple angles (views).
