Our new articles in Communications Biology

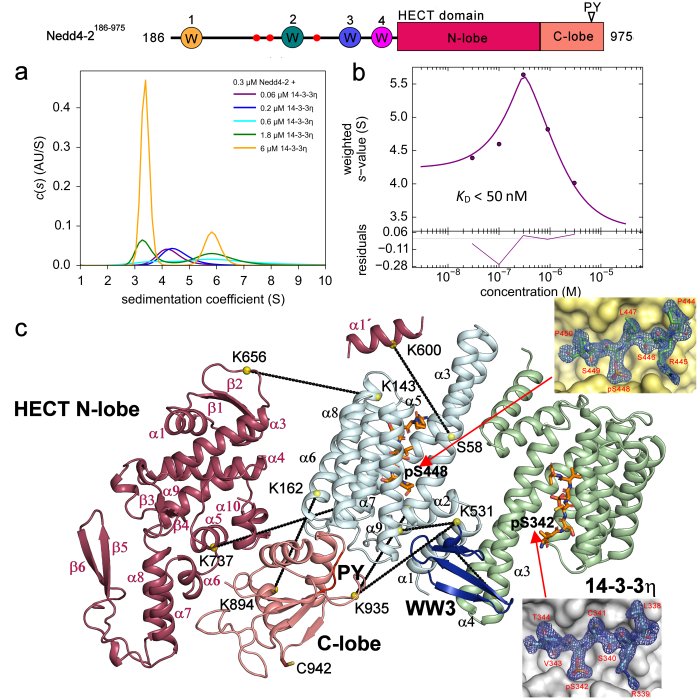
Characterization of the interaction between Nedd4-2 and 14-3-3 in solution. a) Sedimentation coefficient distributions (c(s)) , b) Isotherm of weight-averaged sedimentation coefficients (sw) derived from SV-AUC analysis, c) SAXS-based structural model of the complex with the crystal structures of the Nedd4-2 phosphopeptides (Pohl et al. 2021).
Horvath et al. structurally and biochemically characterized the full-lenght human DAPK-14-3-3 complex to investigate the effects of binding to DAPK2 on its dimerization, activation by dephosphorylation of Ser318, and Ca2+/calmodulin binding. Their results provide mechanistic insights into 14-3-3-mediated DAPK2 inhibition and highlight the potential of the DAPK2:14-3-3 complex as a target for anti-inflammatory therapies.