Clock in the choroid plexus in the brain are sensitive to disruption of the daily regime but resistant to inflammatory conditions (15.4. 2024)
In the ventricles of the brain there is a structure called the choroid plexus where cerebrospinal fluid is produced. Recent research has shown that its role is much broader. It also acts as a barrier for the transfer of substances between the blood and the brain, is involved in the active transport of important substances to the brain, in protecting the brain from neuroinflammation and in the removal of metabolic products of the brain, including those that cause neurodegenerative diseases such as Alzheimer's disease and others. Previous studies have shown that the choroid plexus has a robust circadian clock that controls many of its functions to vary throughout the day and night. In our study, we monitored the clock in the choroid plexus in real time under ex vivo conditions by recording rhythmic changes in bioluminescence that correspond to changes in levels of one of the proteins (PER2) essential for the molecular operation of the clock. We found that disrupting the daily regime in the mouse by altering the light-dark cycle (chronodisruption) not only disrupts the clock in the suprachiasmatic nuclei that controls the sleep-wake cycle, but also significantly suppresses the rhythm of the clock in the choroid plexus. A surprising finding was that, unlike other clocks in the body, the clock in the choroid plexus was completely resistant to the pro-inflammatory state induced by the chronodisruption or by the application of lipopolysaccharide. The mechanism of this resistance is likely related to the specific response of the choroid plexus to chronodisruption at the level of glucocorticoid signaling. The results show high sensitivity of the clock in the choroid plexus to disruption of the circadian system. In addition, they showed that operation of the clock is preserved even during proinflammatory state. These results form the basis for a new direction of research into the impact of disrupted function of the clock in choroid plexus on brain homeostasis.
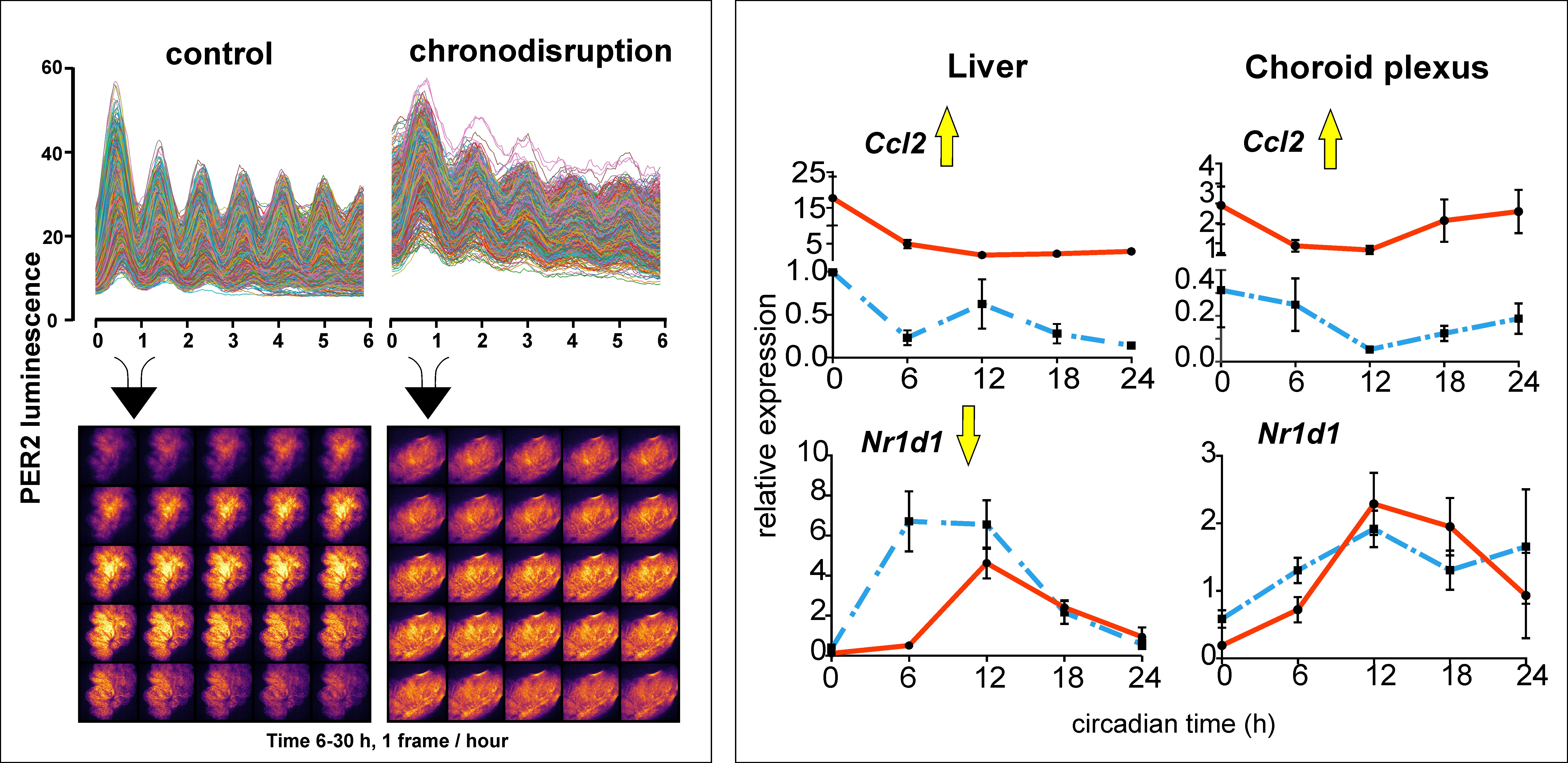
Left: Rhythms of bioluminescence recorded over 6 days with an Olympus LV200 bioluminescence microscope in single cells of the choroid plexus of mPer2Luc mice maintained under control conditions and after exposure to chronodisruption. Right: Differential response of the circadian clock in the liver and choroid plexus to injection of lipopolysaccharide (LPS) in mice. While LPS elicited a pro-inflammatory response (Ccl2) in both tissues, it suppressed the amplitude of rhythmic Nr1d1 clock gene expression only in the liver.
Drapšin M, Dočkal T, Houdek P, Sládek M, Semenovykh K, Sumová A: Circadian clock in choroid plexus is resistant to immune challenge but dampens in response to chronodisruption. Brain Behavior and Immunity. Roč. 117, March (2024), s. 255-269. ISSN 0889-1591. E-ISSN 1090-2139, IF = 15.1 DOI
The ability to transport K+ cations and hence the physiological role of Na+/H+ antiporters is influenced by the composition of their hydrophilic C-termini (31.1. 2024)
It is important for every cell to constantly control the ionic composition of its internal environment (cytoplasm). To cope with the high salt concentrations (e.g. NaCl), cells must be able to eliminate sodium (Na+) cations. On the other hand, the export of potassium cations (K+) from cells is related to the regulation of intracellular pH and membrane potential. Na+/H+ antiporters are among transport systems that ensure the transport of Na+ and K+ out of the cells in exchange for protons in the cells of all eukaryotic organisms, from unicellular yeast to humans. For possible pharmacological intervention in the ion balance, it is necessary to discover, at the molecular level, how the structure of the antiporter impacts its functions.
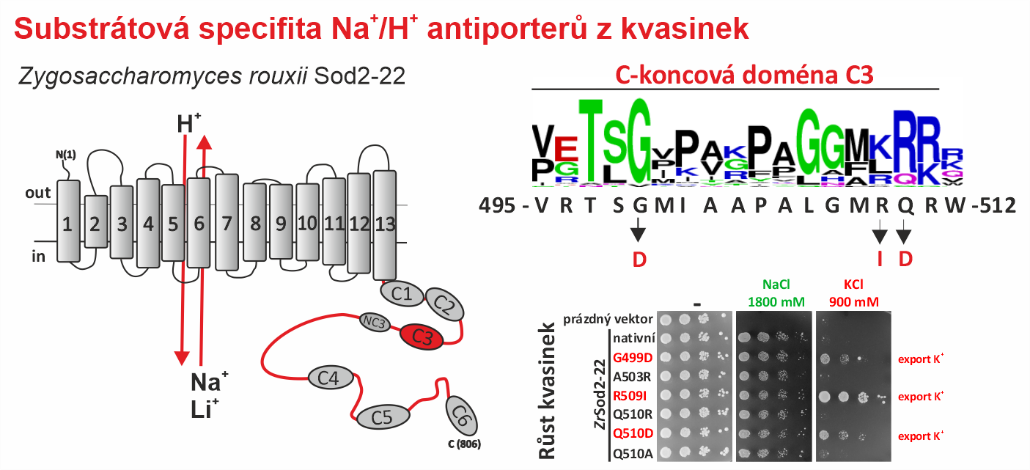
Na+/H+ antiporters, like most membrane proteins, consist of a part located in the membrane and a part oriented to the cytoplasm - the hydrophilic C-terminus (see figure). Until now, it was assumed that only the transmembrane part determines which ions will be transported by the antiporter. In our new work, we demonstrate that the composition of the hydrophilic C-terminus of the antiporter is also important for determining substrate specificity (especially the ability to transport K+).
For the study, we used a single-cell model organism of eukaryotic cells - the yeast Saccharomyces cerevisiae, in which we expressed the Sod2-22 antiporter from the osmotolerant yeast Zygosaccharomyces rouxii, which efficiently exports only Na+ and Li+ cations, but not K+, from the cells. We found that replacing only one amino acid (introducing a negatively charged residue or removing a positively charged residue) in one of the conserved C-terminal regions (C3) enabled the antiporter to transport K+. Truncation or replacement of the C-terminal part of ZrSod2-22 with the C-terminus from another K+-transporting antiporter (S. cerevisiae Nha1 or Z. rouxii Nha1) also resulted in an antiporter with the capacity to export K+. This work provides a number of new insights into the relationship between the structure and function in the Na+/H+ antiporter family in eukaryotic cells.
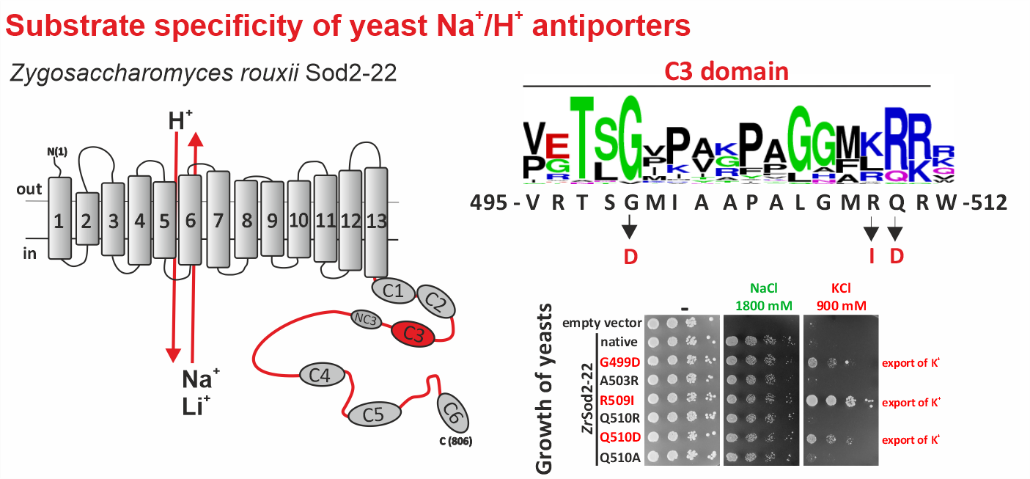
Left: Topological model of Z. rouxii Na+/H+ antiporter Sod2-22 with indicated conserved domains located in the hydrophilic C-terminus. Right: Mutations of particular residues in the conserved domain C3 changed the substrate specificity of the antiporter and enabled it to mediate the export of K+ (demonstrated by the growth of cells expressing these mutated versions in the presence of KCl).
Zimmermannova O., Velazquez D., Papouskova K., Prusa V., Radova V., Falson P., Sychrova H.: The hydrophilic C-terminus of yeast plasma-membrane Na+/H+ antiporters impacts their ability to transport K+. J Mol Biol. 436, 168443 (2024). IF = 5.6 DOI
The "frequency coding" hypothesis provides the classical explanation of the information transmission between nerve cells. This hypothesis relies on the observation that the average rate (frequency) of action potentials increases with stimulation intensity. However, the exact times of action potentials tend to vary even under identical experimental conditions. When evaluating experimental data, or simulated data, we must therefore quantify the variability among multiple trials in order to assess the reliability of frequency coding. The variability of neuronal recordings is usually measured by the Fano factor, whose estimation is considered problematic especially when the amount of data is limited. In this paper, we propose a novel method that allows a precise Fano factor estimation even in situations when the stimulus changes rapidly in time.
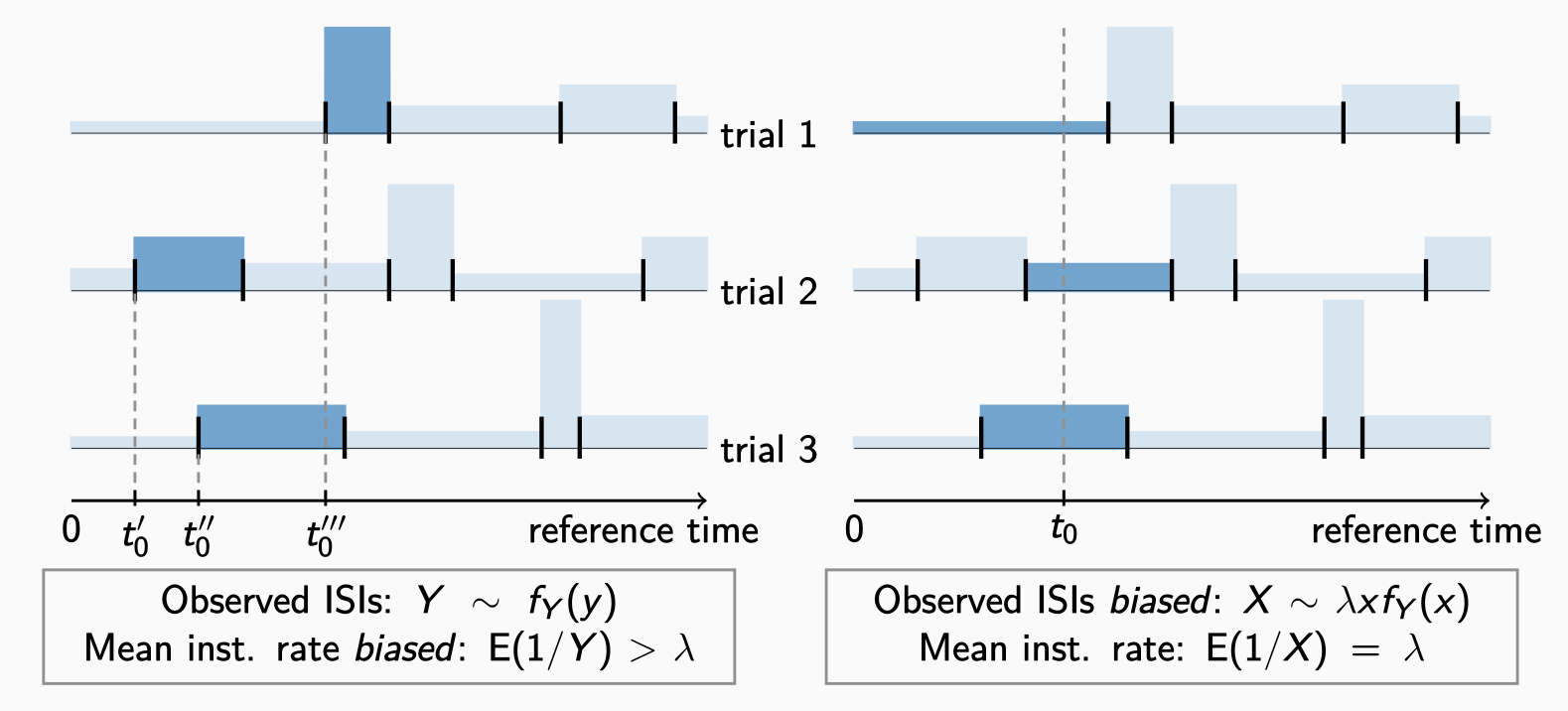
Rajdl K, Košťál L: Estimation of the instantaneous spike train variability. Chaos Solitons & Fractals. Roč. 177, December (2023), 114280. ISSN 0960-0779. E-ISSN 1873-2887 IF: 7.8 DOI
How does 14-3-3 protein block CaMKK protein kinase activity? (16.1. 2024)
CaMKK1 and CaMKK2 kinases regulate key physiological and pathological processes such as tumorigenesis, neuronal morphogenesis, synaptic plasticity, transcription factor activation and cellular energy homeostasis, and promote cell survival. We have elucidated the structural basis of the inhibition of both CaMKK kinases by the regulatory proteins 14-3-3. Our results show that binding of the 14-3-3 protein to both CaMKK1 and CaMKK2 prevents their interaction with calmodulin, a signaling molecule that is essential for their activity. Comparison of the structures of the 14-3-3 complexes with CaMKK also revealed that the catalytic center of CaMKK1 is blocked by the C-terminal helices of the 14-3-3 protein. Our findings may help in the development of new drugs targeted to inhibit CaMKK kinases.
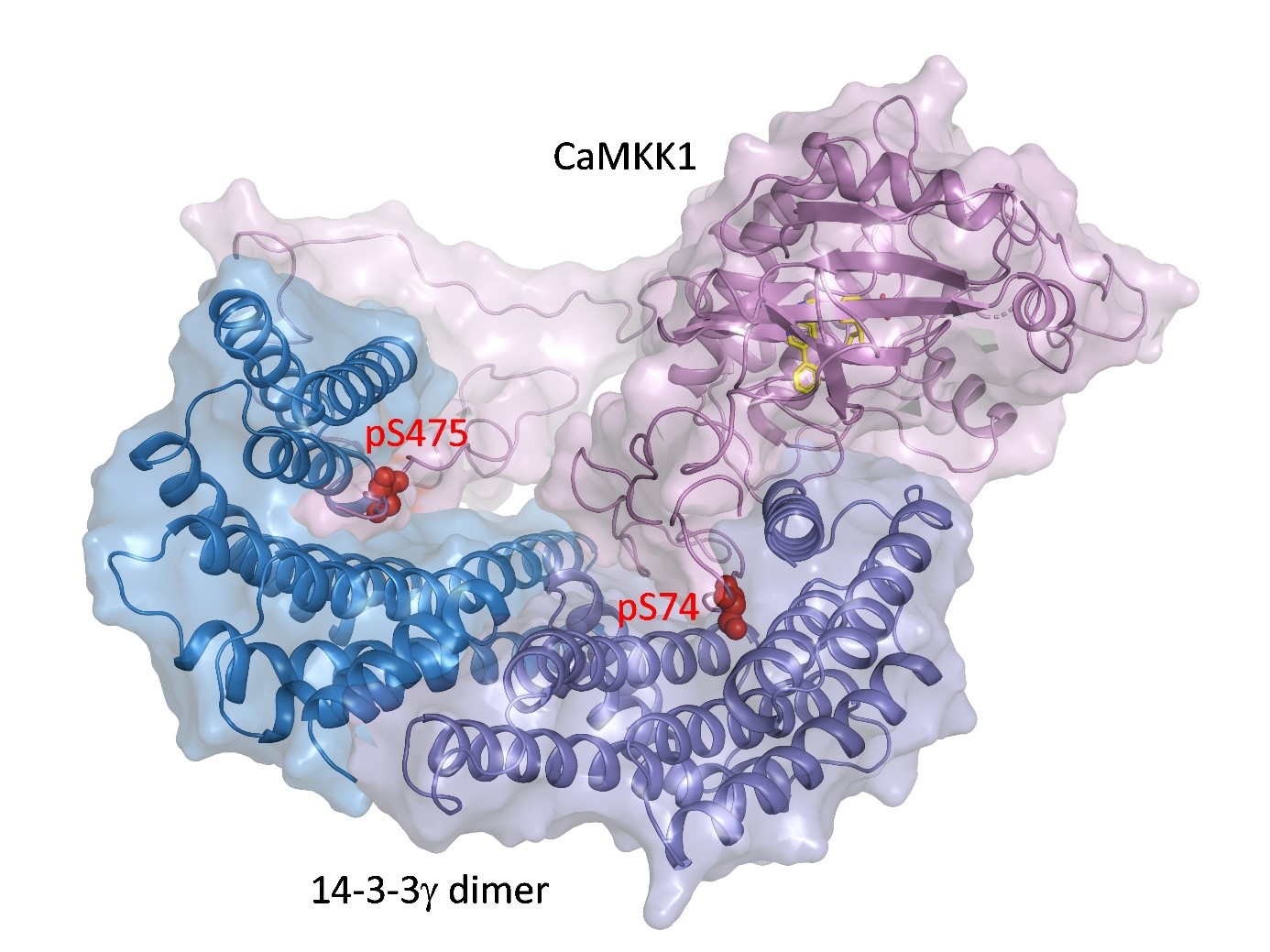
The 14-3-3 protein dimer blocks the catalytic center of CaMKK1 kinase. The kinase active center is shown by the position of the inhibitor (yellow), and the phosphorylation sites of CaMKK1 are shown in red.
Petrvalska O+, Honzejkova K+, Koupilova N, Herman P, Obsilova V*, Obsil T.* 14-3-3 protein inhibits CaMKK1 by blocking the kinase active site with its last two C-terminal helices. Protein Sci. 32 (2023):e4805. ISSN 0961-8368. E-ISSN 1469-896X, IF: 8.000 DOI
+ shared first authorship * shared corresponding autorship
The most common disruption of circadian rhythms in modern society is the so-called social jetlag, i.e. the chronic mismatch between biological time (chronotype) and social time (e.g. waking up according to the alarm clock). However, it is not entirely clear whether social jetlag has any negative effects on health. A large study on a representative adult population of the Czech Republic, conducted by scientists from the Laboratory of Biological Rhythms of the Institute of Physiology of the CAS and published in the journal Sleep, revealed risk factors for the development of cardiovascular and metabolic diseases that are associated with an incorrect sleep schedule.
The authors of the study examined a unique population-representative dataset including 1957 blood samples of adults from across the Czech Republic with different daytime sleep patterns (chronotype). Nine biomarkers (cholesterol, blood lipids, glucose, cortisol, and others) were analyzed in the samples, and an association between social jetlag and biomarkers of cardio-metabolic health (total cholesterol and LDL levels) was found to be statistically significant, especially in people over 50 years of age. The authors also identified new factors influencing social jetlag in the study, such as commuting time to school or work or stress due to lack of time. The study also showed that flexible working hours effectively mitigate social jetlag.
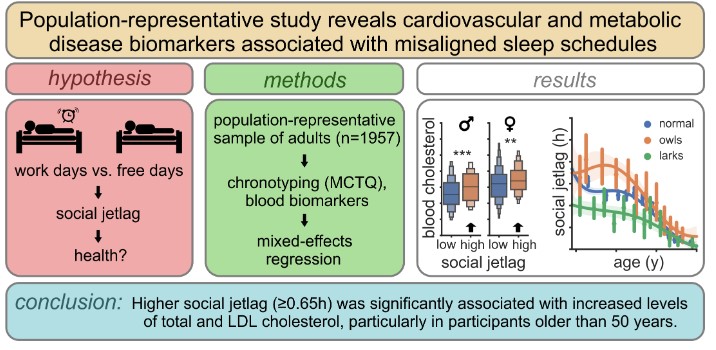
Sládek M, Klusáček J, Hamplová D, Sumová A: Population-representative study reveals cardiovascular and metabolic disease biomarkers associated with misaligned sleep schedules. Sleep, Volume 46, Issue 6, June 2023, zsad037, IF: 5.6 DOI
How do the cells gain enough potassium? (17.5. 2023)
Potassium is an essential intracellular ion, and its optimum intracellular concentration is crucial for many processes; therefore it is fundamental for cells to regulate K+ uptake and efflux via specialised proteins precisely. The Trk1 protein is one of them. Using yeast cells, we showed that a very short part of the protein, the IL2 loop and its two conserved residues (serine 882 and threonine 900) are essential both for the proper folding of the nascent protein and for the regulation of the protein’s activity in response to changes in the extracellular concentration of potassium ions. The binding of regulatory proteins 14-3-3 to phosphorylated threonine 900 is crucial for this regulation.
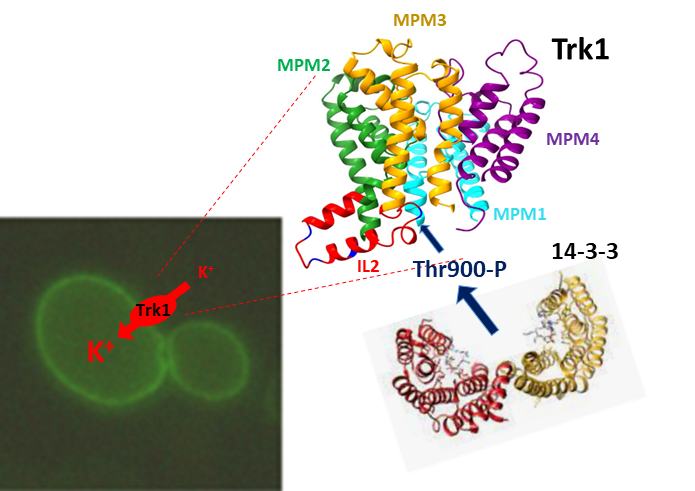
14-3-3 proteins regulate the activity of Trk1 by binding to Thr900 in the second intracellular loop.
Masaryk J, Kale D, Pohl P, Ruiz-Castilla F, Zimmermannová O, Obšilová V, Ramos J, Sychrová H: The second intracellular loop of the yeast Trk1 potassium transporter is involved in regulation of activity, and interaction with 14–3-3 proteins. Computational and Structural Biotechnology Journal. 21 (2023), 2705-2716. ISSN 2001-0370. E-ISSN 2001-0370, IF: 6.155 DOI
Heat from skeletal muscle can protect not only against cold but also against obesity (27.2. 2023)
Press release
Heat production (thermogenesis) is essential for maintaining a constant body temperature, and is an important component of energy balance. Well-described mechanisms involved in heat generation include muscle shivering or non-shivering thermogenesis in brown adipose tissue. Thermogenesis in brown adipose tissue, which is dependent on the presence of the mitochondrial protein UCP1 (uncoupling protein 1), is the focus of interest for its potential use in the treatment of obesity. Other mechanisms of non-shivering thermogenesis and their significance are relatively poorly understood.
Scientists at the Institute of Physiology, Academy of Sciences of the Czech Republic, Prague, investigated heat generation in two different strains of laboratory mice (C57BL/6J and A/J) that differ in their susceptibility to obesity. In C57BL/6J mice, obesity can be induced by a high-fat diet, whereas in A/J mice obesity does not occur under these conditions. Mice of both strains were able to acclimate to cold with only a small decrease in body temperature, initially using muscle shivering for thermogenesis. Prolonged exposure to cold led to activation of thermogenesis in brown adipose tissue in obesity-prone mice. Surprisingly, however, obesity-resistant mice failed to activate brown adipose tissue but increased non-shivering thermogenesis in skeletal muscle. Heat generation in muscle involved increased calcium ion cycling in the endoplasmic reticulum associated with higher mitochondrial oxidative activity.
The involvement of different thermogenic mechanisms could be related to the different susceptibility to obesity. The resistance of A/J mice to obesity is probably due to their ability to activate non-shivering thermogenesis in muscle. These results, published in Molecular Metabolism, also suggest a new possibility for obesity treatment by activating non-shivering thermogenesis in muscle. This is probably a more promising way to treat obesity than potential therapies based on increasing thermogenesis in brown adipose tissue. Especially because in the adult human, the capacity of skeletal muscle to burn fat energy stores is several fold greater than in brown adipose tissue. Thus, only a relatively small increase in thermogenesis in muscle could significantly reduce adipose tissue deposition. How to achieve such an increase is a challenge for further research
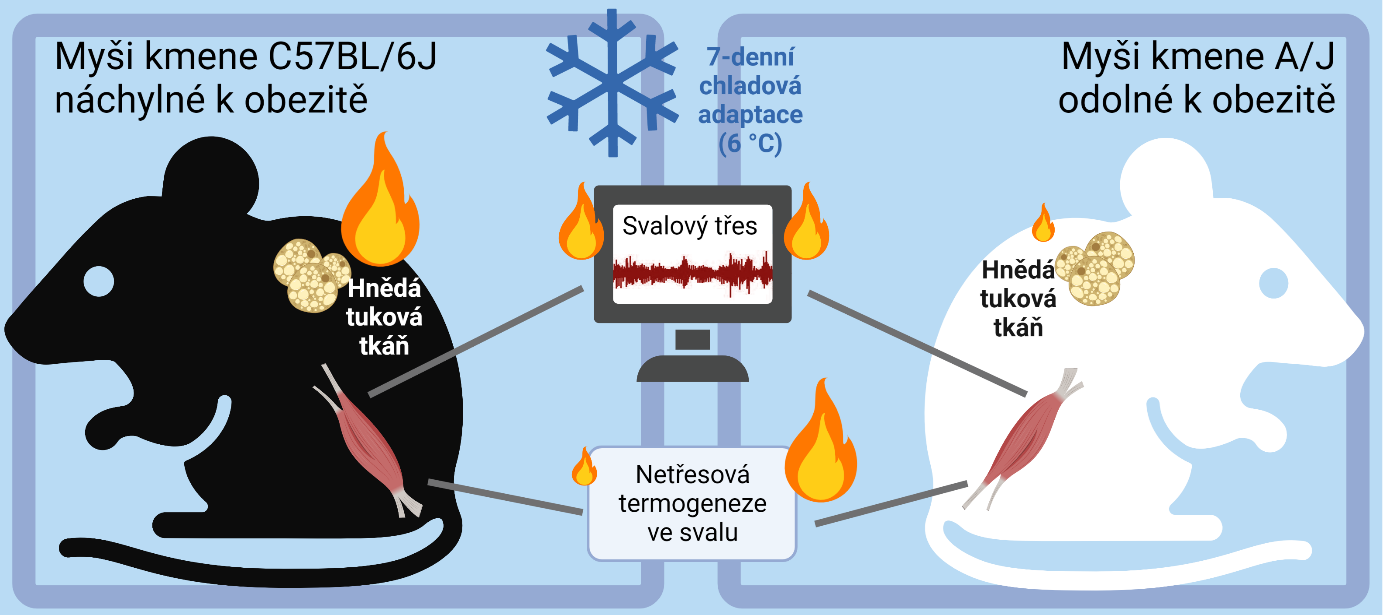
Janovska, P., P. Zouhar, K. Bardova, J. Otahal, M. Vrbacky, T. Mracek, K. Adamcova, L. Lenkova, J. Funda, T. Cajka, Z. Drahota, S. Stanic, A. C. Rustan, O. Horakova, J. Houstek, M. Rossmeisl and J. Kopecky (2023). „Impairment of adrenergically-regulated thermogenesis in brown fat of obesity-resistant mice is compensated by non-shivering thermogenesis in skeletal muscle.“ Mol Metab 69: 101683. IF = 8.568 DOI
Take a pill in the morning or in the evening? The mood stabilizer lithium affects the brain differently depending on the time of day (30.1. 2023)
Press release
Lithium is an effective mood stabilizer, but the mechanism of its therapeutic effect is not well understood. Prof. Sumová's team from the Institute of Physiology of the Czech Academy of Sciences investigated the effect of lithium on the circadian clock located in the ventricular barrier complex containing the choroid plexus, which is a part of the glymphatic system that influences gross brain function via the production of cerebrospinal fluid. They found that treating mice with lithium changed the level of clock gene expression in the choroid plexus. To study the effect of lithium in more detail, they used a transgenic mouse model with a circadian reporter (mPer2LUC) to monitor the choroid plexus clock in real time. When they applied the drug to tissue explanted from the mice in vitro, the effect on the clock was highly dependent on the timing of application. Lithium delayed the clock for most of the day, but during a short window it caused phase advances. Using specific inhibitors, the authors demonstrated that the effect was not mediated via inhibition of the enzyme GSK3, a canonical mechanism of lithium, but rather via modulation of PKC activity. The results suggest a novel mechanism of therapeutic action for lithium that aligns the function of the choroid plexus clock-related glymphatic system with the sleep-wake cycle to improve brain function in psychiatric patients. Importantly, the data argue for personalized timing of lithium treatment in patients with bipolar disorder.
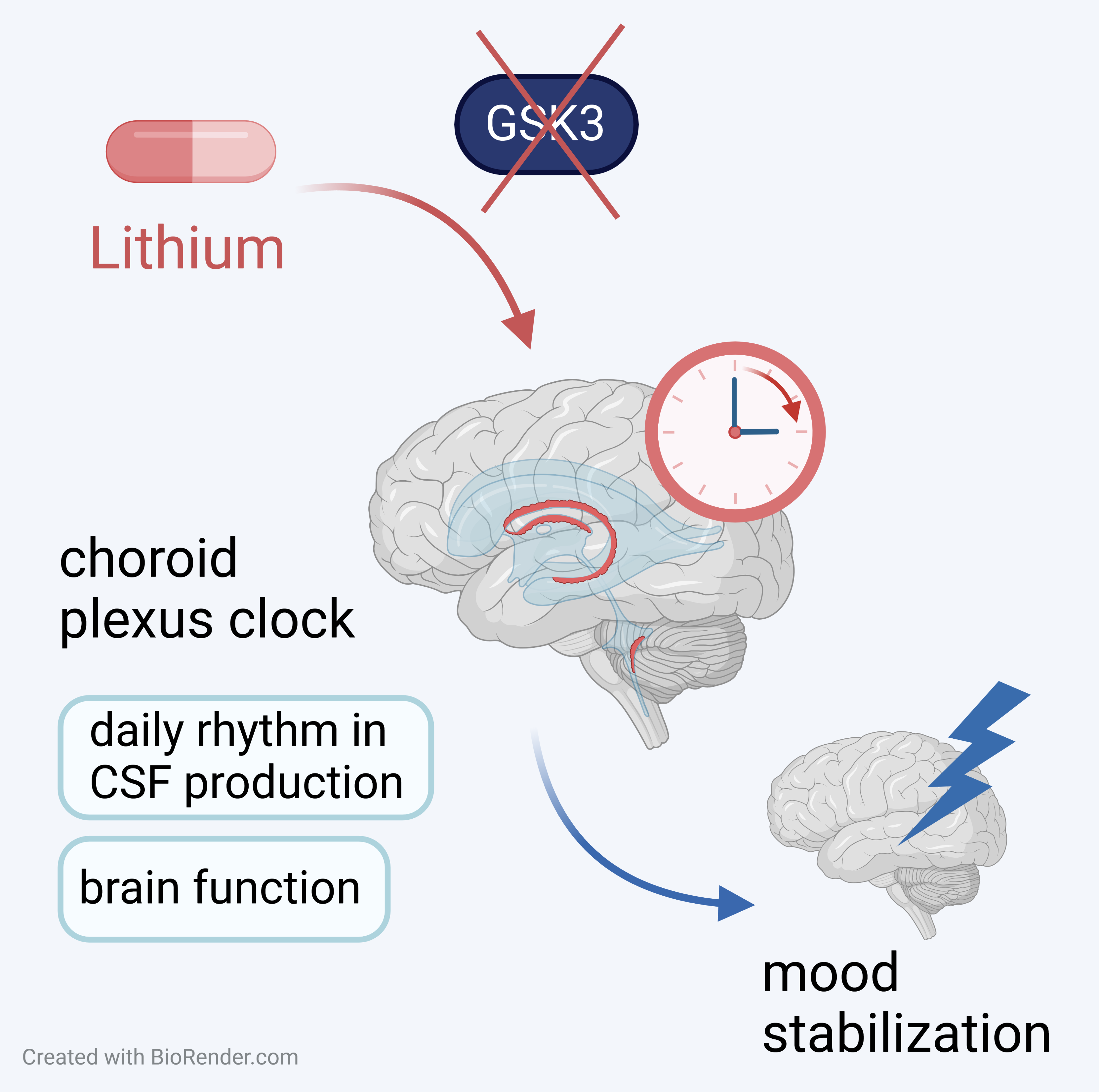
Liška K, Dočkal T, Houdek P, Sládek M, Lužná V, Semenovykh K, Drapšin M, Sumová A: Lithium affects the circadian clock in the choroid plexus – A new role for an old mechanism, Biomedicine & Pharmacotherapy, Vol. 159, March 2023, 114292, IF 7.419 DOI
Heat or cold? New insights into the functioning of temperature-sensitive ion channels obtained in collaboration with Lund University (24.1. 2023)
Temperature-sensitive TRP ion channels are cellular molecular sensors involved in the transduction of sensory signals, pain perception (nociception) and the maintenance of ion homeostasis. Disturbances in their function are associated with many serious human diseases such as chronic pain, inflammation, cancer and various cardiovascular, neurological, respiratory, renal and metabolic disorders. The enormous hope of developing new drugs targeting these channels is greatly limited due to their intrinsic ability to be activated by stimuli of different modalities (polymodality), the mechanism of which has not yet been fully understood. An international team of scientists led by Prof. Peter M. Zygmund (Lund University, Malmö, Sweden), in collaboration with scientists from the Institute of Physiology of the Czech Academy of Sciences in Prague, has revealed two important mechanisms contributing to the polymodal regulation of TRP channels: The first study revealed the binding site and molecular nature of the binding of D9‑tetrahydrocannabiorcol (a natural plant cannabinoid with no psychotropic effects), which strongly facilitates the activation of TRPV2, thereby affecting the transmission of painful stimuli. The second study identified two separate specific regions that confer heat (> 35 °C) and cold (< 15 °C) sensitivity to a different receptor, TRPA1, and demonstrated that the temperature sensitivity of this receptor is critically dependent on oxidative and reducing environments. The results make an important contribution to the understanding of the general molecular mechanisms of chemical and thermal activation of TRP family channels and will find application in the search for possible approaches to their regulation by drugs.
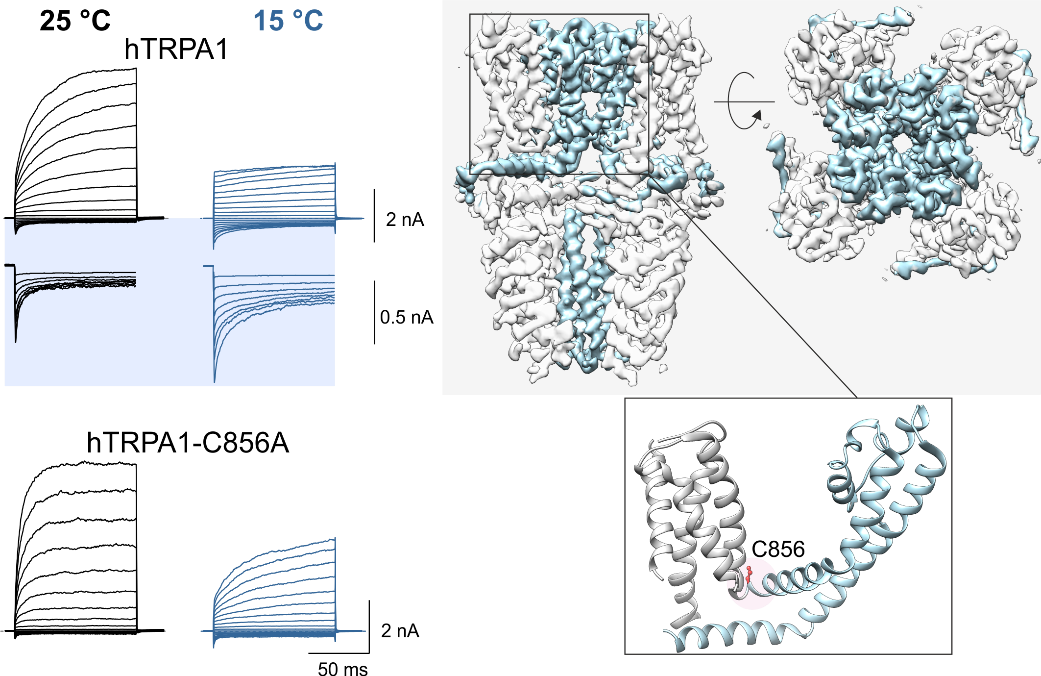
Left panel: effect of cold (15°C) on activation of the human TRPA1 wild-type ion channel and a mutant in which the major amino acid residue C856 responsible for regulation by oxidizing and reducing agents is substituted for alanine. The channels were activated by a series of voltage pulses (from ‑160 mV to +200 mV). Right panel: visualization of the TRPA1 channel structure obtained by cryoelectron microscopy (PDB: 6v9w) as viewed from the side and top. Dynamic regions of the channel responsible for cold activation are highlighted in light blue. The position of cysteine C856 is shown in the detail of the structure of one subunit below.
Moparthi, L. - Sinica, Viktor - Moparthi, V. K. - Kreir, M. - Vignane, T. - Filipovic, M. R. - Vlachová, Viktorie - Zygmunt, P. M.The human TRPA1 intrinsic cold and heat sensitivity involves separate channel structures beyond the N-ARD domain. Nature Communications. Roč. 13, č. 1 (2022), IF: 17.694 DOI
Zhang, L. - Simonsen, Ch. - Zímová, Lucie - Wang, K. - Moparthi, L. - Gaudet, R. - Ekoff, M. - Nilsson, G. - Hellmich, U. A. - Vlachová, Viktorie - Gourdon, P. - Zygmunt, P. M. Cannabinoid non-cannabidiol site modulation of TRPV2 structure and function. Nature Communications. Roč. 13, č. 1 (2022), IF: 17.694, rok: 2021 DOI
Unravelling novel regulatory mechanisms in the human Na+/H+ antiporter NHA2 (14.11. 2022)
Sodium/proton antiporters are membrane proteins found in all living cells from bacteria to humans and play an important role in regulating intracellular pH and cation concentrations. Among these types of transporters belong also the NHA2 antiporter, whose activity affects a number of physiological functions, e.g. insulin secretion, sodium reabsorption in the kidneys or sperm motility. The NHA2 antiporter transports Na+ or Li+ cations across the membrane in exchange for H+ and its activity is specifically inhibited by phloretin. The properties and functions of all proteins result from their primary structure, i.e. from the sequence of amino acids from which the protein consists. In the family of Na+/H+ antiporters, NHA2 has a unique structure. It consists of 537 amino acids with 14 transmembrane domains and an unique hydrophilic N-terminus long 82 amino acid residues, whose structure and function have not yet been studied. Our new results published in the Protein Science journal revealed several new structural elements important for the NHA2’s function, including the unravelling a new regulatory role of the hydrophilic N-terminus.
We studied the human NHA2 protein and its mutated variants using its expression in a model eukaryotic organism, the yeast Saccharomyces cerevisiae, as well as using bioinformatic simulations (in collaboration with the laboratory of Prof. Nir Ben-Tal from Tel-Aviv University). We newly identified several amino acid residues important for antiporter selectivity (recognition and transport of Na+ and Li+ cations) and for transport of protons. Furthermore, we determined the place in the protein structure where the phloretin inhibitor binds. We also revealed that the unique hydrophilic N-terminal part of the protein has an important (autoinhibitory) role in regulating the transport activity of NHA2, because truncations of the first 50 - 70 residues of the N-terminus doubled the transport activity of the antiporter. Our results also show that the new expression system for NHA2 in yeast cells can be useful for a rapid screening of SNP´s effects on NHA2 activity, and/or to test new compounds influencing the function of NHA2, similarly as phloretin.
Velazquez D., Prusa V., Masrati G., Yariv E., Sychrova H., Ben-Tal N. and Zimmermannova O. (2022): Allosteric links between the hydrophilic N-terminus and transmembrane core of human Na+/H+ antiporter NHA2. Protein Sci: e4460. IF = 6.993 DOI
Load next










