How does 14-3-3 protein block CaMKK protein kinase activity?
CaMKK1 and CaMKK2 kinases regulate key physiological and pathological processes such as tumorigenesis, neuronal morphogenesis, synaptic plasticity, transcription factor activation and cellular energy homeostasis, and promote cell survival. We have elucidated the structural basis of the inhibition of both CaMKK kinases by the regulatory proteins 14-3-3. Our results show that binding of the 14-3-3 protein to both CaMKK1 and CaMKK2 prevents their interaction with calmodulin, a signaling molecule that is essential for their activity. Comparison of the structures of the 14-3-3 complexes with CaMKK also revealed that the catalytic center of CaMKK1 is blocked by the C-terminal helices of the 14-3-3 protein. Our findings may help in the development of new drugs targeted to inhibit CaMKK kinases.
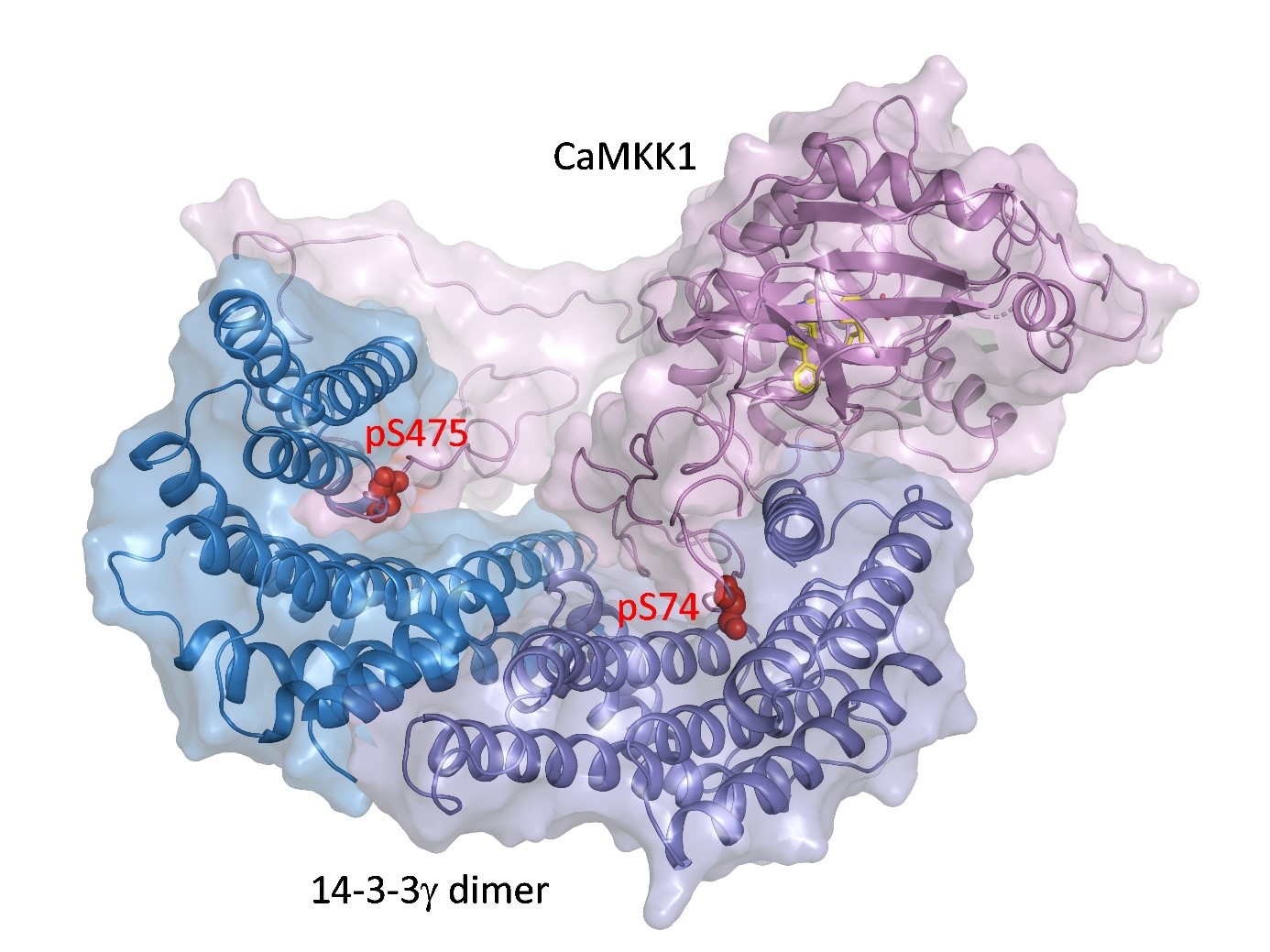
The 14-3-3 protein dimer blocks the catalytic center of CaMKK1 kinase. The kinase active center is shown by the position of the inhibitor (yellow), and the phosphorylation sites of CaMKK1 are shown in red.
Petrvalska O+, Honzejkova K+, Koupilova N, Herman P, Obsilova V*, Obsil T.* 14-3-3 protein inhibits CaMKK1 by blocking the kinase active site with its last two C-terminal helices. Protein Sci. 32 (2023):e4805. ISSN 0961-8368. E-ISSN 1469-896X, IF: 8.000 DOI
+ shared first authorship * shared corresponding autorship
