Elucidation of molecular basis of 14-3-3 protein-dependent activation of trehalase Nth1 in yeast cells
The research team of Veronika Obsilova from the Institute of Physiology CAS and Tomas Obsil from the Faculty of Science, Charles University deciphered the mechanism by which the 14-3-3 protein regulates the function of yeast neutral trehalase Nth1.
Neutral trehalase Nth1 catalyzes the hydrolysis of disaccharide trehalose and represents one of the key enzymes in the energetic metabolism of yeast. The results of their research, which was performed solely in the Czech Republic, were now published by the prestigious American scientific journal Proceedings of the National Academy of Sciences of the USA (PNAS).
„Solved atomic structure of the 14-3-3 protein complex with neutral trehalase Nth1 shows the ability of the 14-3-3 protein to modulate the structure of a multidomain enzyme and to function as an allosteric regulator. It is the second solved structure of 14-3-3 protein complex with the fully active enzyme,“ says Veronika Obsilova.
The scientists from Prague solved the crystal structures of several forms of neutral trehalase Nth1 and deciphered the changes which occur upon the ligand and 14-3-3 protein binding. They showed that the catalytic activity of Nth1 is enabled by the proper 3D configuration of Nth1´s catalytic and calcium-binding domains relative to each other, which stabilizes the flexible part of the active site required for catalysis. This finalized their long-lasting research focused on understanding of the mechanism of yeast neutral trehalase regulation.
„We were able to decipher the structural basis of the 14-3-3 dependent activation of the enzyme Nth1. Comparison of the 14-3-3:Nth1 complex structure with those of other 14-3-3 complexes highlights the ability of 14-3-3 to modulate the structure and function of many client proteins important for the regulation of key cell processes,“ explains Veronika Obsilova.
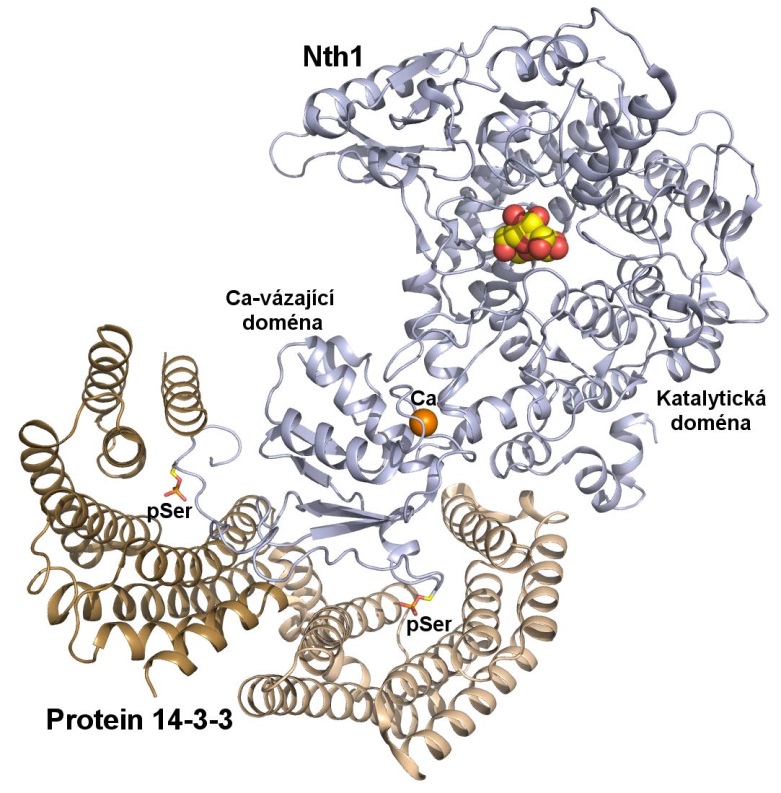
Detailed insight on structure of the complex 14-3-3:Nth1 (PDB 5M4A).
Alblova M, Smidova A, Docekal V, Vesely J, Herman P, Obsilova V, Obsil T. Molecular basis of the 14-3-3 protein-dependent activation of yeast neutral trehalase Nth1. Proc Natl Acad Sci U S A. 2017 Nov 14;114(46):E9811-E9820. IF = 9.66.
