Antidiabetic lipid mediators as neutral lipase substrates
Fat mass is controlled by the balance of triacylglycerol (TAG) degradation and synthesis. Adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) are key players in TAG catabolism providing fatty acids (FAs) as energy substrates and metabolic intermediates. In collaboration with colleagues from University of Graz, Université de Montpellier and Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, we discovered that ATGL and HSL metabolize TAGs containing antidiabetic lipid mediators (FA esters of hydroxy FAs), distinctly controlling the release of bioactive lipids. Our paper connects lipolysis-mediated TAG metabolism with the regulation of antidiabetic signaling lipids.
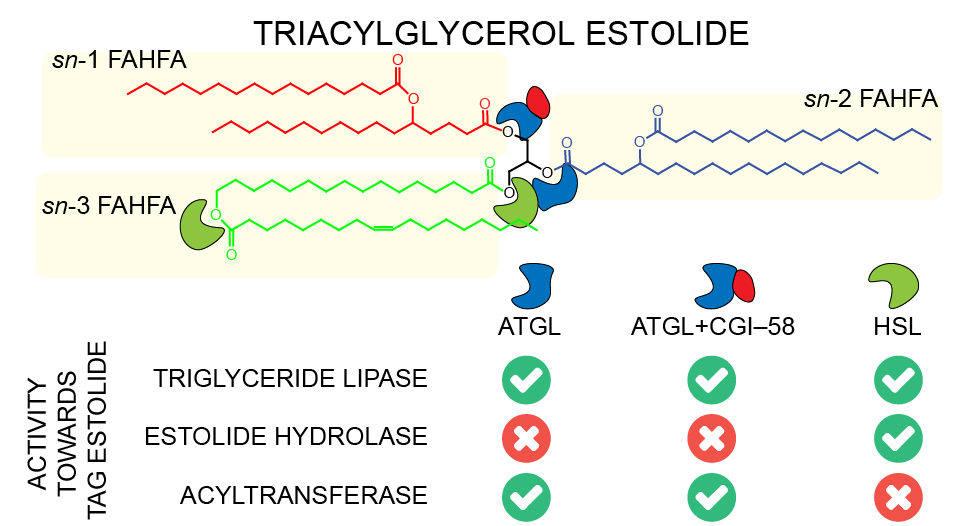
Glycerol-bound FAHFAs in TAG, named TAG estolides, serve as metabolite reservoir of FAHFAs mobilized by lipases upon demand. We found that ATGL alone or co-activated by comparative gene identification-58 (CGI-58) efficiently liberated FAHFAs from TAG estolides with a preference for more compact substrates where the estolide branching point is located near the glycerol ester bond. ATGL was further involved in transesterification and remodeling reactions leading to the formation of TAG estolides with alternative acyl compositions. HSL represented a much more potent estolide bond hydrolase for both TAG estolides and free FAHFAs. FAHFA and TAG estolide accumulation in white adipose tissue of mice lacking HSL argued for a functional role of HSL in estolide catabolism in vivo.
K. Brejchova, F.P.W. Radner, L. Balas, V. Paluchova, T. Cajka, H. Chodounska, E. Kudova, M. Schratter, R. Schreiber, T. Durand, R. Zechner, O. Kuda. Distinct roles of adipose triglyceride lipase and hormone-sensitive lipase in the catabolism of triacylglycerol estolides. Proceedings of the National Academy of Sciences of the United States of America 118(2) (2021) e2020999118. IF = 9.412 DOI
