Functional effects of human disease-associated NMDAR gene mutations
It is estimated that nearly 1% of the global population is affected by some form of autism spectrum disorder, 2% by intellectual disability, 2% by attention deficit hyperactivity disorder, and 1-1.5% by schizophrenia. Neurodevelopmental and neuropsychiatric disorders are strongly linked to mutations of the glutamatergic signalling complex.
Genome-wide association studies have identified a significant number of single nucleotide variants in genes of the NMDAR signalling complex, including many de-novo mutations located in NMDAR exons. While over 700 patient-derived NMDAR gene variants have been identified, the functional consequences of the majority of them are unknown, and only a handful of the known disease-associated NMDAR mutations have been studied in neuronal preparations or animal models. Filling this gap would significantly enhance our knowledge of NMDAR function and could suggest treatment strategies.
We use various methods to elucidate the functional effects of NMDAR mutations found in children diagnosed with epilepsy, intellectual disability or autism. We use electrophysiology, structural biology, biochemistry and microscopy to study mutated NMDARs in heterologous expression systems. We also work with transgenic animal models (zebrafish, mouse) to assess the effects of NMDAR mutations on synaptic transmission, neuronal network activity, and animal behavior.
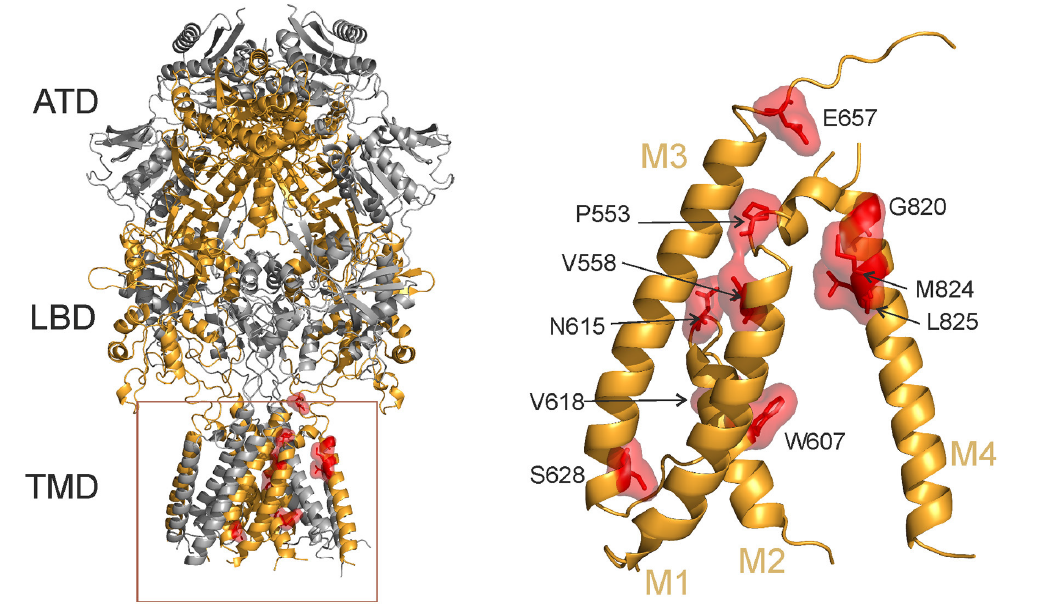
Locations of rare mutations in the transmembrane domain of the GluN2B subunit of human NMDAR. Ribbon structure of a homology model of the hGluN1/hGluN2B receptor (hGluN1 in grey; hGluN2B in orange). Selected residues representing amino acids that were altered in human patients are highlighted in red. ATD = amino-terminal domain; LBD = ligand-binding domain; TMD = transmembrane domain; M1, M3, M4 = transmembrane helices; M2 = re-entrant pore loop.
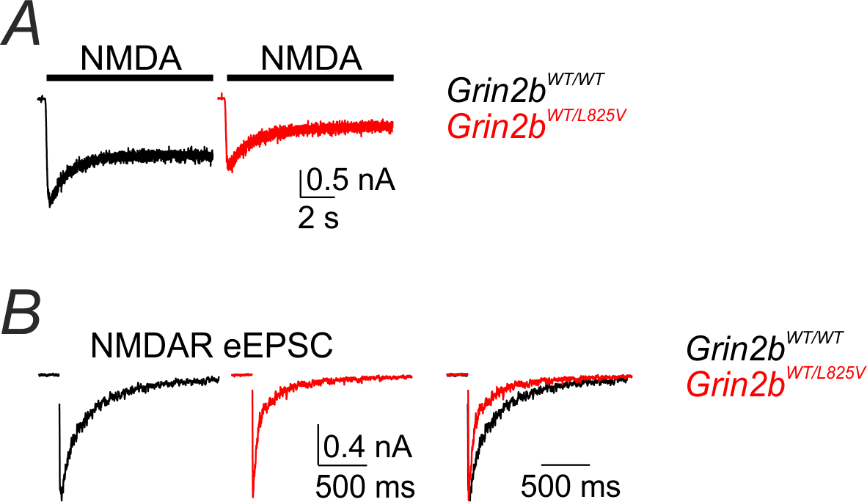
A single allele of an NMDAR gene variant identified in a patient with autism alters NMDAR signaling in neurons. Neurons prepared from heterozygous Grin2bWT/L825V mice have smaller NMDA-evoked currents (A) and shorter NMDAR-mediated synaptic responses (B) compared to neurons prepared from Grin2bWT/WT mice.
